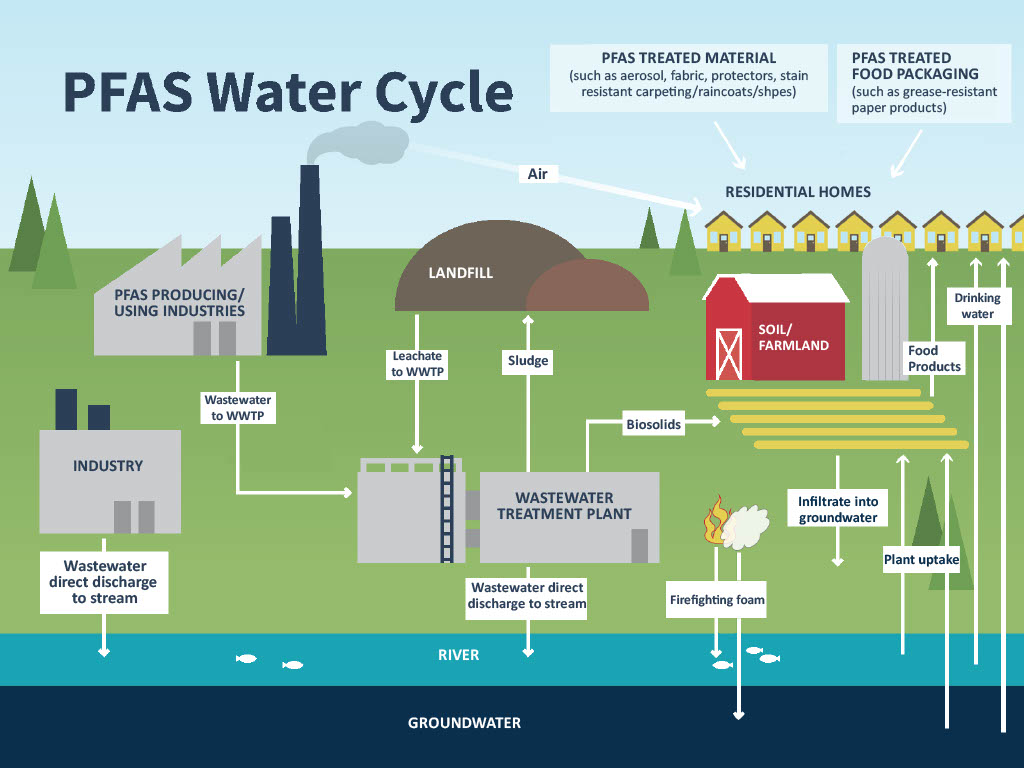
PFAS are a complex group of synthetic chemicals consisting of carbon-fluorine bonds which are used across industries and in primary and secondary consumer products due to their thermal and chemical stability and water and stain resistance.
With the regulatory liabilities and exposure related challenges and toxicology surrounding PFAS, both Nationally and Internationally, RHP Risk Management experts can provide assistance and guidance with this family of chemicals regulatory and exposure related challenges including:
- Regulatory Compliance
- use, waste
- Health and Safety Audits
- manufacturer, use
- Product Labeling
- origin, use, distribution
- Safety Data Sheet Evaluation
- Creation of Hazard Summaries
- Exposure Modeling and Monitoring
- Human Health Risk Assessment
- Formula Review of Products
- Historic Waste Processes and Secondary Use
- State of the Knowledge
RHP experts continue to follow and provide guidance in addressing regulatory updates including:
- Toxic Substances Control Act (TSCA) Reporting and Recordkeeping Requirements for PFAS.
- EPA Final PFAS National Primary Drinking Water Regulation.
- FDA Modernization of Cosmetics Regulation Act of 2022 (MoCRA).
Additionally, RHP scientists have joined the efforts of an international team of researchers, regulators, industry, and academia in performing human health risk assessment evaluations on a variety of PFAS compounds.
The first compound assessed was PFOA, see Dr. Frank Pagone’s publication reporting on a safe dose range. Follow RHP’s Drs. Frank Pagone and Ashish Jachak and this acclaimed team, the Alliance for Risk Assessment (ARA), as they tackle the compound PFOS.