
Most people are familiar with every day units and likely do unit conversions without even thinking about it, such as feet to inches, or cups to ounces. More complex conversions like miles to kilometers or pounds to kilograms may still be commonplace. However, what about complex scientific units? The average coffee drinker may know they prefer two packets of sugar per cup, but what if instead of packets per cup, they had to find their preferred sugar concentration in nanograms-per-deciliter? And then convert that number to parts per million? Further what if they had to find the concentration of all the different potential sugar types like glucose, sucrose, and fructose? If information was presented in such complex detail at coffee shops, people would order a lot more black coffee.
The same is true of scientific writing targeted at the general public. PFAS related articles in particular bombard readers with chemical names and scientific jargon that can leave readers in a state of panic or confusion. Answering the following questions can provide context for risk presented in articles, and help readers identify similarities and points of difference in various published sources:
What language is used to refer to PFAS?
“PFAS” is a general term to describe a group of chemicals that includes all per- and poly-fluoroalkyl substances, however language is not standardized across published media, and academic studies often investigate a specific subset of the many chemicals that fall under the PFAS category. PFOA (Perfluorooctanoic Acid) and PFOS (Perfluorooctane Sulfonate) are two of the most prevalent variants of PFAS compounds, however the distinction between individual chemicals and groups of chemicals is critical for understanding risk. For example, “total exposure estimates for PFAS” may present higher values than “total exposure estimates for PFOA” because the former includes significantly more chemicals in the PFAS category, where the latter includes a single specific chemical. In this case, higher values may or may not imply more risk than lower values and vice versa, because of inclusive or exclusive grouping.
What units are PFAS concentrations presented in, and are all units present on the same scale?
PFAS is often measured and presented in different units depending on the substance in which the PFAS level is being measured. For example, PFAS in drinking water is measured in parts-per-trillion (PPT), in solid foods or animal tissue in micrograms-per-kilogram (ug/kg), and in human blood in micrograms-per-deciliter (ug/dL) or nanograms-per-milliliter (ng/mL). These units are not all on the same scale, meaning the same numeric value can appear much larger or much smaller depending on the unit, and can mislead readers who are drawing conclusions from the reported numeric values.
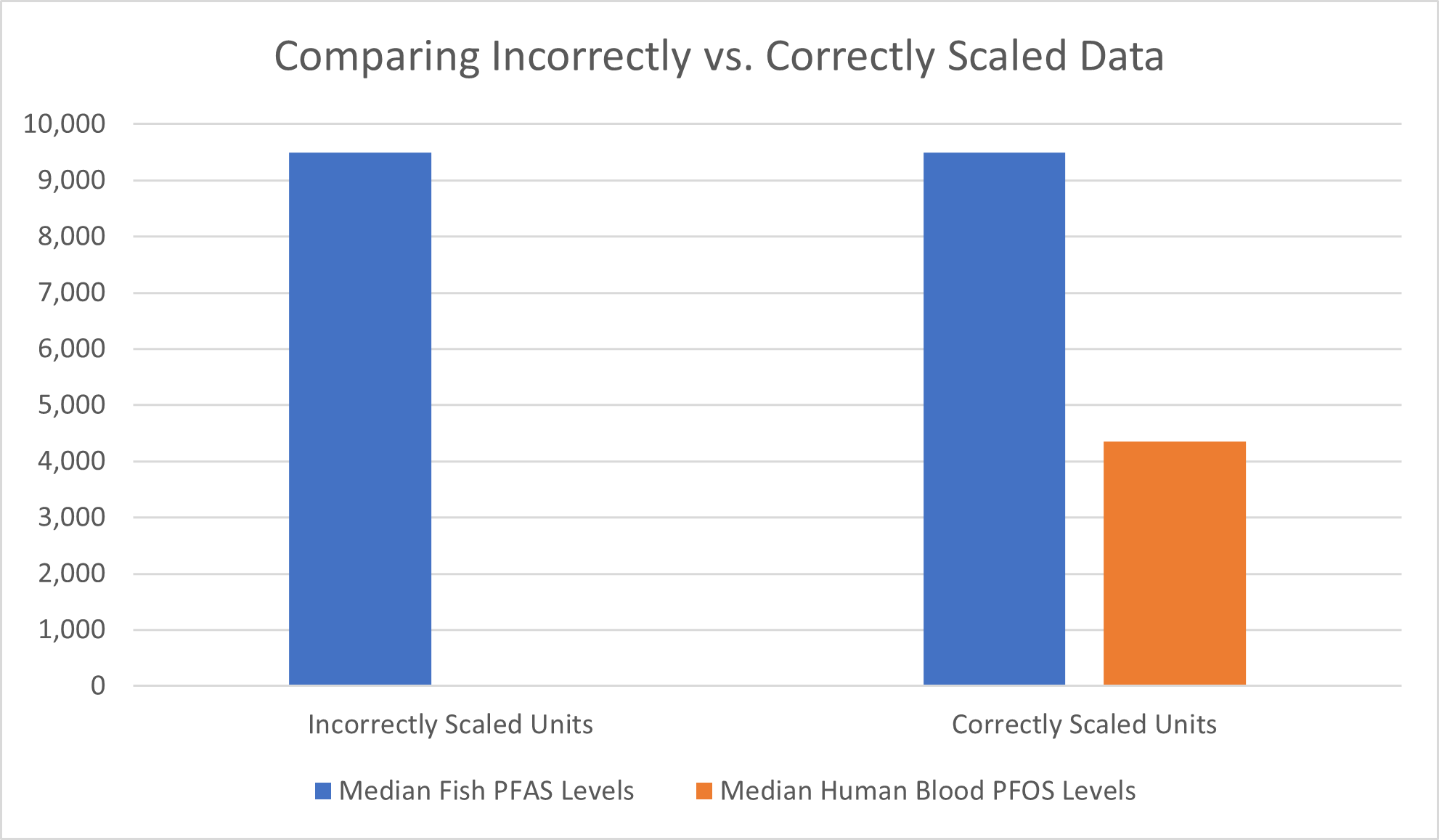
It is important to be mindful of the units that important statistics such as the median are presented in, as studies may report multiple median values for PFAS in the same study. A recent national source news article directed readers to a previous study in which median values of PFAS in fish were reported at 9,500 ng/kg, and later in the same study it was reported that the median PFOS concentration in human serum samples was 4.35 ng/mL. At first glance, these numbers appear alarming because the concentrations the study report in fish (9,500 ng/kg) appears to be almost two thousand times higher than the concentrations the study report in humans (4.35 ng/mL). However, looking closer one sees the units are not on the same order of magnitude, and the values reported for fish include a universe of PFAS chemicals, where the values reported in humans specifically measured only PFOS. If the human serum concentration is converted from ng/mL to ng/L, a unit on the same order of magnitude as ng/kg, the value is 4,350 ng/L, which drastically changes the narrative in comparison to 9,500 ng/kg. The disparity between the two values would likely continue to shrink if the 4,350 ng/L value included the remaining chemicals in the PFAS category rather than just PFOS.
Is there a PFAS reference concentration?
While the previous two points help readers gauge magnitude of risks and put values into context, they do not provide guidance for decision making. Understanding that freshwater fish may contain PFAS chemicals in tissue at double the concentration of humans is meaningless without a reference point. What concentrations are deemed safe for consumption by medical literature? What are the target concentrations for food products or human serum? Are these values higher or lower than the regulatory standard? It is critical to have a reference point, or some point which at or below risk is reduced to an acceptable level. Additionally, reference points must also be in the appropriate unit to compare to a given concentration.
For more information on RHP’s services and contact information, please contact Frank Pagone, PhD, CIH at fpagone@rhprisk.com, (773) 867-6011 or Ben Heckman, MPH, CIH at bheckman@rhprisk.com, (717) 706-3847 and visit rhprisk.com.
